Anti-Infective Therapeutics
At Zymeron, our anti-infective therapeutics (AIT) program focuses on discovering new drug targets and novel mechanisms of action and developing novel compounds and formulation strategies that can better mitigate the antimicrobial drug resistance problem. Drug resistance is a consequence of infectious agent evolution in part responding to biological pressures imposed on these microorganisms. Our AIT technologies take aim at reducing the likelihood of drug resistance development and minimizing the probability of microorganisms to genetically pass the drug resistance traits down during replication. Our AIT program focuses on 1) Targeted Protein Degradation (TPD) technologies; 2) Pathogen-agnostic therapeutics; and 3) Host-defense biomimetic strategies.
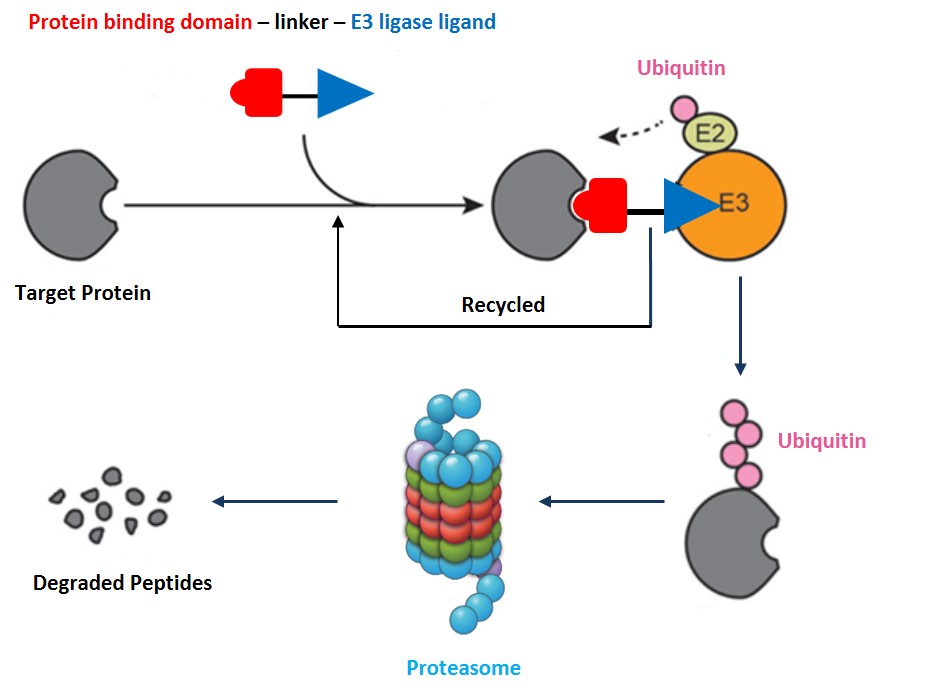
Targeted Protein Degradation
Targeted protein degradation is a disruptive drug technology that has the potential to enable game changing antiviral therapeutic capabilities. The principle of this novel strategy utilizes chimeric, tripartite molecules that bind to the target protein including those that are previously undruggable, triggering its polyubiquitination by ubiquitin-activating enzymes, ubiquitin-conjugating enzymes, and ubiquitin ligases. The polyubiquitin protein tag marks the substrate protein for subsequent degradation in the proteasome. The chimera compounds, small molecule protein degraders, orchestrate the “kiss of death” for the target protein. Contrary to traditional small molecule inhibitors, protein degraders offer the advantage of completely abrogating all catalytic, structural and scaffolding functions of the protein. Protein degraders only need transient binding to induce ubiquitination and therefore require lower binding affinity and are less susceptible to point mutations. Our protein degraders target viral and host proteins that are crucial to virion entry and replication, or chronic infection. These protein degraders are developed as the next generation antivirals for viruses that either lack functional cure or have widespread drug resistance problems.
Current TPD programs develop Proteolysis Targeting Chimeric (PROTAC) Antivirals for hepatitis B and human immunodeficiency virus:
- Anti-HIV targeting accessory proteins that disrupt host immunity and enhance infectivity
- Anti-HBV targeting cccDNA transcription to achieve functional cure of HBV infections